East China’s Jiangsu province will step up its efforts to promote the high-quality development of the pharmaceutical industry in accordance with the Action Plan on Optimizing Review and Approval Services to Promote the Use of Innovative Drug Devices and Promote the High-Quality Development of the Pharmaceutical Industry between 2022 and 2024, the general office of the provincial government said at a press conference held Tuesday afternoon.
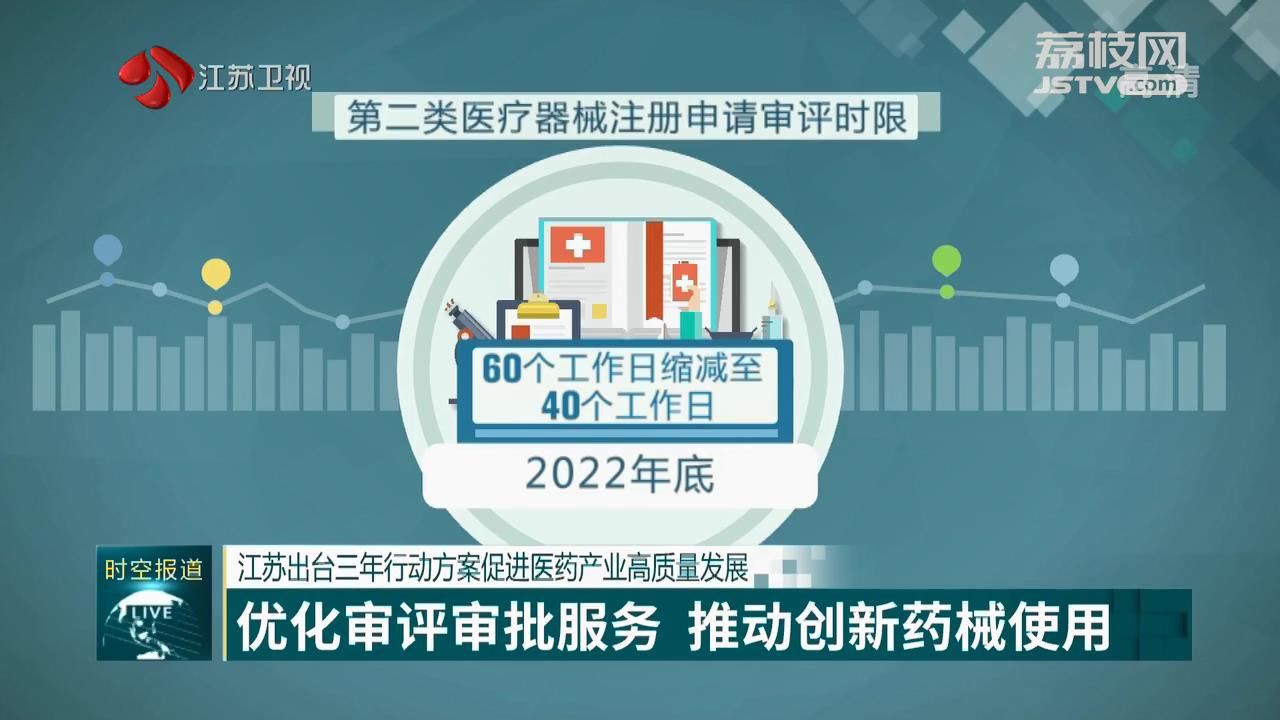
The action plan proposes specific work goals to be achieved every year before the end of 2024 by focusing on optimizing the review and approval time range and improving the review and approval service system.
Before the end of this year, Jiangsu will reduce the time spent on reviewing second-class medical devices such as blood glucose meters from 60 working days to 40 working days.
Before the end of 2023, Jiangsu will reduce the time for drug registration inspection from 60 working days to 45 working days, and by the end of 2024, it will be further reduced to 30 working days.

Tian Feng, director of the Jiangsu Provincial Food and Drug Administration, was quoted as saying that the time reduction was achieved by implementing process re-engineering, expanding review and approval resources, and improving work efficiency on the basis of strictly implementing review and approval standards and ensuring product quality and safety.
The goal-oriented action plan proposes 12 specific measures around review and approval, clinical trial research, the use of innovative medical devices, and promoting industrial quality and efficiency to ensure that the tasks are completed on time.
At the same time, the action plan clearly states that a multi-departmental collaborative working mechanism must be established to further form a synergy to promote the high-quality development of the pharmaceutical industry.





