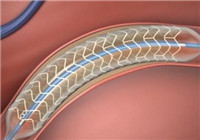
The State Drug Administration has approved the registration of "bio-absorbable coronary rapamycin eluting stent system", which means that the country’s first domestic bio-absorbable cardiac stent will be officially put into use to refrainmetal stent from being used in the patient's blood vessels.
The product consists of a stent and a delivery system, wherein the matrix of the stent and the drug carrier coating are respectively made of the absorbable material L-polylactic acid and racemic polylactic acid, and the stent substrate and the coating can be gradually biodegraded and absorbed in the body with no permanent stent to remain in the patient’s blood vessels.
This product is the first bio-resorbable stent in China for the treatment of intravascular stenosis in patients with primary coronary atherosclerosis, and will offer more clinical treatment options for patients with coronary heart disease.
According to the "China Cardiovascular Disease Report 2017", there are 290 million cardiovascular patients in China, including 11 million patients with coronary heart disease. Interventional therapy is currently one of the most effective methods for the treatment of coronary heart disease.
(source:ourjiangsu.com)